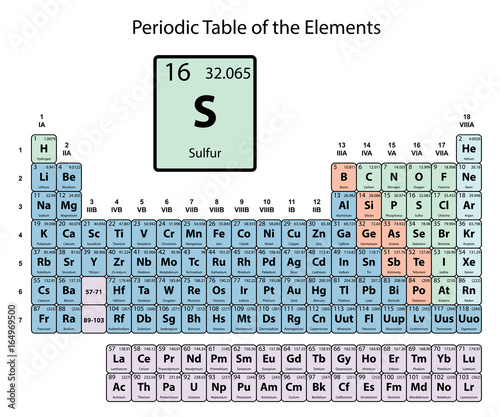
We elaborate the uses of sulfur and atomic properties with characteristics. Sulfur is a lemon-yellow chemical element with an atomic number 16. Its symbol is S and it belongs to the group of nonmetals and its usual state in nature is solid. Sulfur is located at position 16 on the periodic table.
You Can Visit Our Managed: Periodic Table Main Page
The atomic radius of Sulfur atom is 105pm (covalent radius). Atomic Number: 16 Atomic Mass: 32.066 amu Melting Point: 112.8 °C (385.95 K, 235.04001 °F) Boiling Point: 444.6 °C (717.75 K, 832.28 °F) Number of Protons/Electrons: 16 Number of Neutrons: 16 Classification: Non-metal Crystal Structure: Orthorhombic Density @ 293 K: 2.07 g/cm 3 Color: yellow British Spelling: Sulphur IUPAC Spelling: Sulfur. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that. Name: Sulfur: Symbol: S: Atomic Number: 16: Atomic Mass: 32.066 atomic mass units: Number of Protons: 16: Number of Neutrons: 16: Number of Electrons: 16: Melting.
Racepak usb devices driver download for windows 10. On this page you can discover the chemical properties of sulfur and information about sulfur and other elements on the periodic table such as oxygen, selenium, phosphorus or chlorine. You will also learn what sulfur is for and know its uses through its properties associated with sulfur such as its atomic number or the usual state in which sulfur can be found.
You will be able to see sulfur qualities such as its melting and boiling point, its magnetic properties or what its chemical symbol is. In addition, here you will find information about its atomic properties such as the distribution of electrons in sulfur atoms and other properties.

For some elements, some of this information is unknown. In these cases we show the properties attributed to them.
Sulfur properties
One of the properties of non-metal elements such as sulfur is for example that non-metal elements are poor conductors of heat and electricity. Sulfur, like the other non-metal elements, has no luster. Due to their brittleness, nonmetals such as sulfur cannot be flattened to form sheets or stretched to become threads.
The state of sulfur in its natural form is solid. Sulfur is a lemon yellow chemical element and belongs to the group of non-metals. The atomic number of sulfur is 16. The chemical symbol for sulfur is S. The melting point of sulfur is 388.36 degrees Kelvin or 116.21 degrees Celsius or degrees Celsius. The boiling point of sulfur is 717.87 degrees Kelvin or 445.72 degrees Celsius or degrees Celsius.
Uses of sulfur
Sulfur is a bright yellow crystalline solid that is essential for life. If you’ve ever wondered what sulfur is for , here is a list of its possible uses:
- Most sulfur is converted to sulfuric acid. Sulfuric acid is extremely important to many industries around the world. It is used in the manufacture of fertilizers, petroleum refining, wastewater treatment, batteries lead for cars, ore mining, removal of oxide iron , nylon manufacturing and production of hydrochloric acid.
- Sulfur can be used as a pesticide and fungicide. Many farmers who grow organic food use sulfur as a natural pesticide and fungicide.
- Sulfur-containing magnesium sulfate is used as a laxative, in bath salts, and as a magnesium supplement for plants.
- Sulfur is important to life. Therefore, it is added to fertilizers (in soluble form) so that plants have more sulfur available in the soil.
- Carbon disulfide , a sulfur compound, can be used to make cellophane and rayon (a material used in clothing).
- Sulfur is used to vulcanize rubber. Rubber vulcanization makes it more difficult. It ensures that the rubber maintains its shape. Vulcanized rubber is used to make car tires, shoe soles, hoses, and ice hockey pucks.
- Other sulfur compounds (sulfites) are used to bleach the paper and preserve the fruit.
- Sulfur is also a component of gunpowder.
Atomic properties of sulfur
The atomic mass of an element is determined by the total mass of neutrons and protons that can be found in a single atom belonging to this element. As for the position where to find sulfur within the periodic table of the elements, sulfur is in group 16 and period 3. Sulfur has an atomic mass of 32,065 u.
The electronic configuration for sulfur is [Ne] 3s2 3p4. The electronic configuration of the elements, determines the form in which the electrons are structured in the atoms of an element. The average radius of sulfur is 100 pm, its atomic radius or Bohr radius is 88 pm, its covalent radius is 102 pm, and its Van der Waals radius is 180 pm. Sulfur has a total of 16 electrons whose distribution is as follows: In the first layer it has 2 electrons, in the second it has 8 electrons and in its third layer it has 6 electrons.
You Can Visit Our Managed: Periodic Table Main Page
Sulfur characteristics
Below you can see a table showing the main characteristics of sulfur.
| Sulfur | ||
|---|---|---|
| Chemical symbol | S | |
| Atomic number | 16 | |
| Group | 16 | |
| Period | 3 | |
| Appearance | lemon yellow | |
| Block | p | |
| Density | 1960 kg / m3 | |
| Atomic mass | 32,065 u | |
| Average radius | 100 pm | |
| Atomic radio | 88 | |
| Covalent radius | 102 pm | |
| Van der Waals radio | 180 pm | |
| Electronic configuration | [Ne] 3s2 3p4 | |
| Electrons per layer | 2, 8, 6 | |
| Oxidation states | + -2,4,6 (strong acid) | |
| Crystal structure | orthorhombic | |
| State | solid | |
| Melting point | 388.36 K | |
| Boiling point | 717.87 K | |
| Heat of fusion | 1.7175 kJ / mol | |
| Vapor pressure | 2.65 × 10-20Pa at 388 K | |
| Electronegativity | 2.58 | |
| Specific heat | 710 J / (K · kg) | |
| Electric conductivity | 5.0 × 10-16S / m | |
| Thermal conductivity | 0.269 W / (Km) | |
What Is The Atomic Number Of Sulfur 4
You Can Visit Our Managed: Periodic Table Main Page
Drivers silicon integrated hard disk controller. Watch video to know more information about Sulphur.
Demi Moore is an actress, producer, director and activist. She is known for her roles in St. Elmo's Fire, About Last Night, Ghost, A Few Good Men, Indecent Proposal, G.I. Jane, Charlie's Angels: Full Throttle, Margin Call, among many others. Amazon inside out demi moore. Inside Out is a story of survival, success, and surrender - a wrenchingly honest portrayal of one woman’s at once ordinary and iconic life. Read more Read less ©2019 Demi Moore (P)2019 HarperAudio. Inside Out by Demi Moore is a 2019 Harper publication. A compelling and revealing memoir I waffled back and forth on this one. Initially, I talked myself out of reading it, then changed my mind when I saw it had generated a little buzz and I noticed most of the reviews were positive. Demi Moore is an actress, producer, director and activist. She is known for her roles in St. Elmo’s Fire, About Last Night, Ghost, A Few Good Men, Indecent Proposal, G.I. Jane, Charlie’s Angels: Full Throttle, Margin Call, among many others. Moore is also a co-founder of Thorn, a non-profit that builds technology to defend children from sexual abuse, exploitation, and trafficking. Demi Moore is an actress, producer, director and activist. She is known for her roles in St. Elmo’s Fire, About Last Night, Ghost, A Few Good Men, Indecent Proposal, G.I. Jane, Charlie’s Angels: Full Throttle, Margin Call, among many others.
How many valence electrons are in an atom of sulfur?
1 Answer
Explanation:
Sulfur has six valence electrons.
Atomic Number Of Sulfur 35
Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels. Consequently, these are the electrons available for chemical bonding.

To determine the valence number, look at electron configurations which denote the number of electrons in the different energy levels and orbitals.
Neutral sulfur has 16 electrons because its atomic number is 16.
The electron configuration is
What does this configuration tell us?
Sulfur has two electrons in the
The next energy level, the last one, is the outermost energy which comprises the valence shell. There are two electrons in the
Related questions
